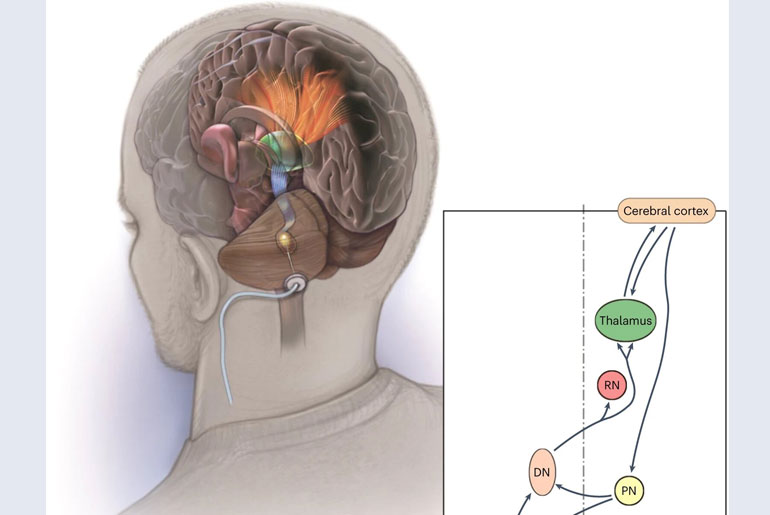
Researchers from the Cleveland Clinic have conducted a groundbreaking first-in-human trial of deep brain stimulation (DBS) for post-stroke rehabilitation patients. The trial focused on targeting the dentate nucleus in the brain, which plays a crucial role in regulating fine control of voluntary movements, cognitive functions, language, and sensory abilities.
Named the EDEN trial (Electrical Stimulation of the Dentate Nucleus for Upper Extremity Hemiparesis Due to Ischemic Stroke), the study demonstrated the safety and feasibility of using DBS for stroke rehabilitation. The trial also revealed that a majority of participants (nine out of 12) exhibited improvements in both motor impairment and overall function.
One noteworthy finding from the study was that participants who had at least minimal preservation of distal motor function at the beginning of the trial experienced remarkable gains, nearly tripling their initial scores. The results, published in Nature Medicine, built upon more than a decade of preclinical work led by researchers Andre Machado, M.D., Ph.D., and Kenneth Baker, Ph.D., at the Cleveland Clinic.
“These are reassuring for patients as the participants in the study had been disabled for more than a year and, in some cases, three years after stroke. This gives us a potential opportunity for much needed improvements in rehabilitation in the chronic phases of stroke recovery,” stated Dr. Machado, chair of Cleveland Clinic’s Neurological Institute. “The quality-of-life implications for study participants who responded to therapy have been significant.”
The DBS method used in stroke recovery was patented by Dr. Machado, and the Vercise DBS systems utilized in the trial were provided by Boston Scientific, which holds a license to these patents. Cleveland Clinic Innovations established Enspire DBS Therapy, Inc., a portfolio company, to commercialize the method, and co-funded the study. Dr. Machado holds stock options and equity ownership rights with Enspire and serves as the chief scientific officer.
“We saw patients in the study regain levels of function and independence they did not have before enrolling in the research,” Dr. Machado stated. “This was a smaller study, and we look forward to expanding as we have begun the next phase.”
The EDEN trial involved 12 individuals who had experienced chronic, moderate-to-severe hemiparesis of the upper extremity due to a unilateral middle cerebral artery stroke 12 to 36 months prior to the study. The trial yielded no major complications. Participants underwent DBS surgery, during which electrodes were implanted into the cerebellum. These electrodes were connected to a device similar to a pacemaker, delivering controlled electric pulses to aid in restoring movement control.
“The safety and feasibility data from this early study combined with the potential symptom improvements certainly support the need for additional, larger trials to see if cerebellar DBS is indeed a potential treatment for post-stroke motor impairment,” stated Brooks Gross, Ph.D., program director, National Institute of Neurological Disorders and Stroke.
Following surgery and recovery, participants engaged in months of physical therapy, initially with the DBS device turned off for several weeks and then activated for four to eight months. The most significant improvements were observed after the device was turned on.
“There are currently no effective methods to improve the outcomes of physical rehabilitation for the hundreds of thousands of stroke survivors,” stated Dr. Baker, Cleveland Clinic Lerner Research Institute. “The results of the study found that deep brain stimulation, paired with physical therapy, improved movement in patients who were more than a year out from their stroke and whose motor improvements had largely plateaued. This tells us the research warrants further investigation in larger patient samples.”
This groundbreaking study showcases the potential of DBS as a post-stroke rehabilitation tool, offering new hope for individuals recovering from stroke-related impairments.
Disclaimer:
The information contained in this article is for educational and informational purposes only and is not intended as a health advice. We would ask you to consult a qualified professional or medical expert to gain additional knowledge before you choose to consume any product or perform any exercise.